PureDireX - PDC01-0100
PCR Clean-Up & Gel Extraction Kit|PureDireX
Size: 100 rxns
PureDireX PCR Clean-Up & Gel Extraction Kit
The PCR Clean-Up & Gel Extraction Kit provides a cost-effective system for the fast and easy isolation of the DNA fragments from PCR reactions, agarose gels, or enzymatic reactions. The DNA fragments (100bp~10Kb) in the special buffers are bound by the glass fiber matrix of the spin column while contaminants pass through the column. Impurities are efficiently washed away, and the pure DNA is eluted with the Tris buffer or water without phenol extraction or alcohol precipitation. The DNA purified with the kits is suitable for any subsequent application, such as ligation and transformation, sequencing, restriction enzyme digestion, labeling, PCR, in vitro transcription, or microinjection. The entire procedure can be completed within 15~20 minutes.
Fastest Procedure: Completed within 30mins
Sample: up to 100μl of PCR Product or 300 mg of Agarose Gel
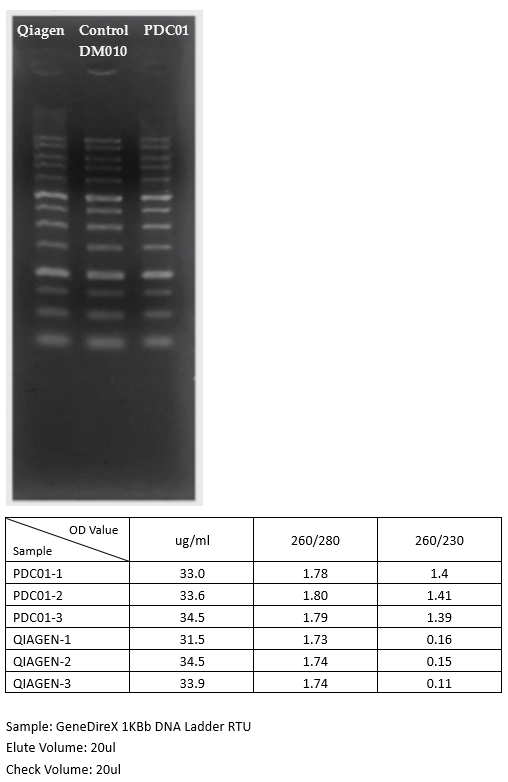
Recovery: up to 95%
[PureDireX / PDC01-0100 / Bio-Helix]
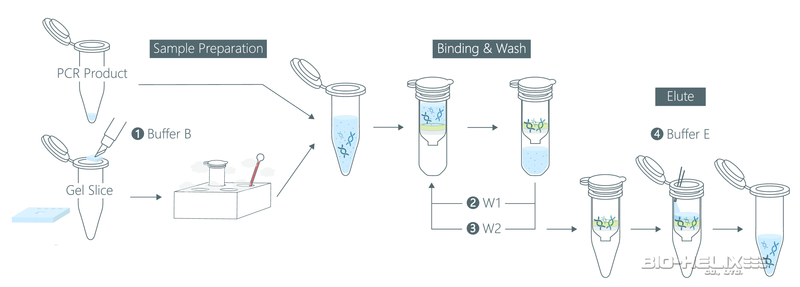
Step 1 - Sample Preparation
PCR Clean Up
1. Add 500 μl of the Buffer B to 100 μl of the PCR product and mix by vortex.
Gel Extraction
1. Excise the DNA fragment from the agarose gel.
2. Transfer up to 300 mg of the gel slice to a 1.5 ml microcentrifuge tube.
3. Add 500 μl of the Buffer B to the sample and mix by vortex. Incubate at 60°C for 10 minutes
(or until the gel slice has completely dissolved).
4. During the incubation, mix by vortexing the tube every 2~3 minutes.
5. Cool the dissolved sample mixture to the room temperature.
Step 2 - Binding
1. Place a PG Column in a Collection Tube. Apply the supernatant (from step 1) to the PG Column by decanting or pipetting.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the PG Column back into the same collection tube.
*The maximum volume of the PG Column reservoir is 800 μl. If the sample mixture is more than 800 μl, repeat the DNA Binding Step.
Step 3 - Wash
1. Add 400 μl of the Buffer W1 into the PG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the PG Column back into the same collection tube.
4. Add 600 μl of the Buffer W2 (ethanol added) into the PG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the PG Column back into the same collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove the residual Buffer W2.
Step 4 - Elution
1. To elute the DNA, place the PG Column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 μl of the Buffer E or H2O (pH is between 7.0 and 8.5) to the center of each PG Column, let it stand for 2 minutes, and centrifuge at 14,000 x g for 2 minutes.
*Check the buffers before use for salt precipitation. Redissolve any precipitate by warming to 37°C.
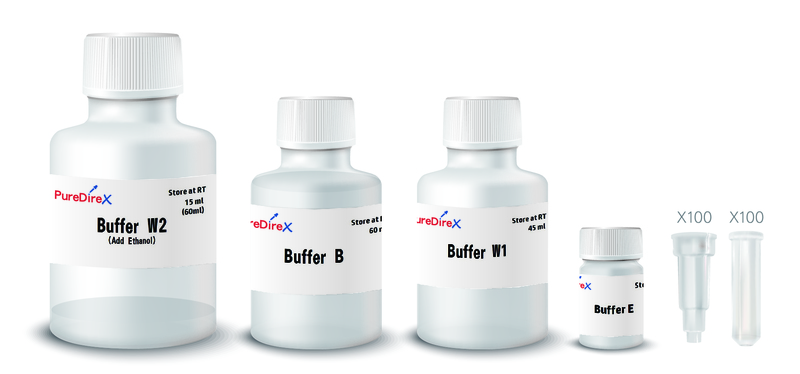
Buffer B: 60 ml
Buffer W1: 45 ml
Buffer W2: 15 ml (Add 60 ml of the ethanol (96~100%) to the Buffer W2 before use)
Buffer E: 10 ml
PG Columns: 100 pcs
Collection Tubes: 100 pcs
| Name | Download |
|---|---|
| PureDireX_MSDS_PDC01-0100 | PureDireX_MSDS_PDC01-0100.pdf |
| PureDireX_PDC01-0100_Protocol | PureDireX_PDC01-0100_Protocol_V1_2024.pdf |