LifeDireX - CCH1108
UltraScenceCLIA FemtoPlus Western Substrate
Size: Custom fill

Bio-Helix has been dedicated to developing bio-reagents for laboratory research in the past decade. Bio-reagents play a critical role in the life science research as well as in the diagnosis development. We decided to extend our efforts into the IVDD (in-vitro diagnostic device) market as it is best to intervene with disease progressing before its outbreak. With our knowledge and innovative technology, we want to help patients around the world for the early detection of illness in order to receive proper treatment and maintain better life quality. Disease may never be eradicated. However, early discovery leads to early treatment, thus a better chance to cure.
It is therefore our aim to develop an array of diagnostic products to be used at both bedside and bench side. Whether it is a pathological, complex or inheritance disease, Bio-Helix will bring products beneficial to the life and health circles.

Enhanced Chemiluminescent Substrate
With different unique enhancers, its limit of detection can achieve up to the femto-gram level.
Application
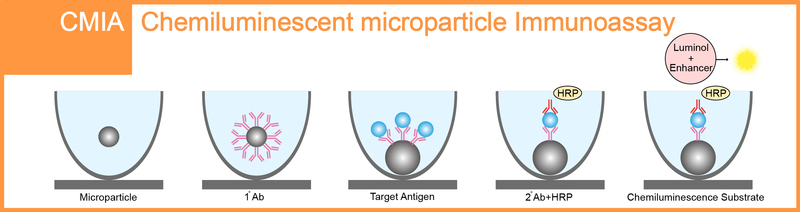
Qualified for Chemiluminescent Immunoassay & Chemiluminescent Microparticle Immunoassay.
|
Key Facts • Limit of detection (LOD) can be tailor-made. • The product format can be tailor-made. |
|---|
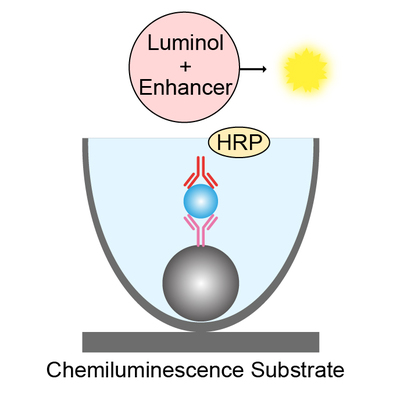
Chemiluminescence immunoassay (CLIA) is an assay that combines the chemiluminescence technique with immunochemical reactions, which is available for the detection and analysis of antigens, antibodies, enzymes, and drugs etc. In recent years, CLIA has gained increasing attention in various fields such as clinical diagnosis, environmental monitoring, and pharmaceutical analysis because of its safety, high sensitivity, and good specificity etc.
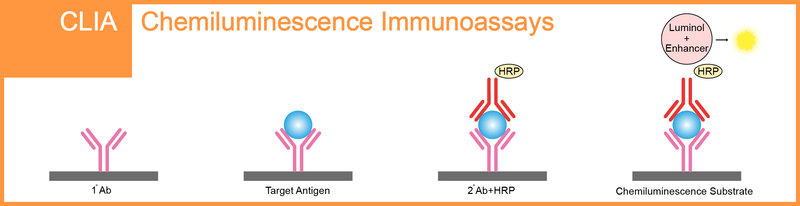
Depending on different luminescence mechanisms, CLIA includes direct chemiluminescence immunoassay, chemiluminescence enzyme immunoassay, and electrochemiluminescence immunoassay. Regarding chemiluminescence enzyme immunoassay, an enzyme, such as horseradish peroxidase (HRP), is first used to label an antigen or antibody, after immunoreaction, luminol as a luminescent substrate emits light by oxidation-reduction reaction under the action of the enzyme-labeled immune-reactant and the luminescent reagent, and then the antigen or antibody is determined by luminescent intensity.
Although light emission generated rapidly by the aforementioned reaction, the duration of luminescence is short, resulting in that the stability and reproducibility of the detection results are not good enough. Subsequent studies have found that the use of one or more chemical agents (enhancers) can increase the luminescent intensity and prolong the luminescence time.
Our reagent for performing a chemiluminescent reaction, which can not only effectively enhance the luminescent intensity, but also greatly increase the luminescence time.
**US Patent No.: US10,711,185
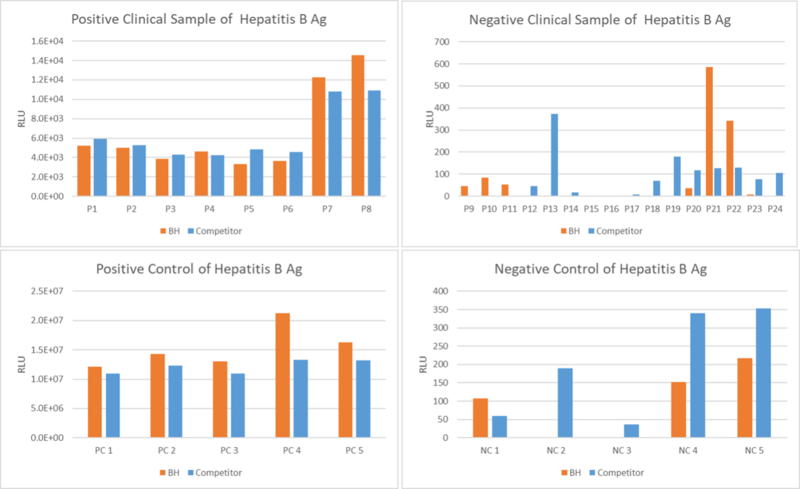
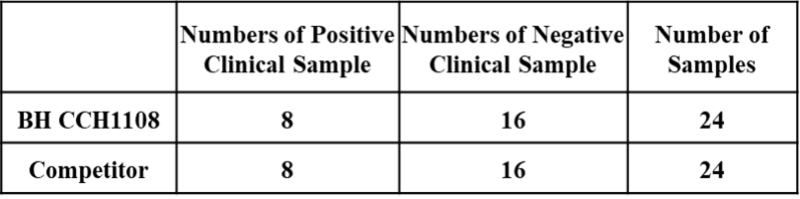
Figure 1. An assay was performed to assess the Hepatitis B Ag clinical sample by chemiluminescence immunoassay (CLIA). Testing of 24 clinical samples for the Qualitative/Quantitative determination and compared side by side with the competitor. The assay was tested on Dia. Pro instrument named SARA.
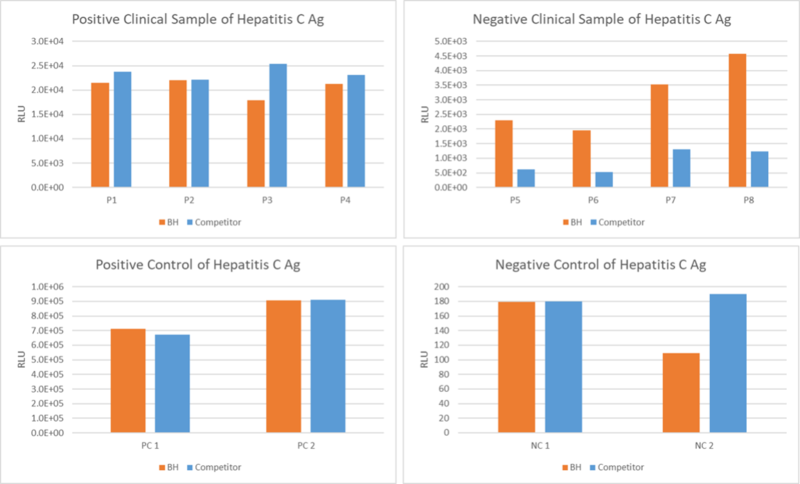

Figure 2. An assay was performed to assess the Hepatitis C Ag clinical sample by chemiluminescence immunoassay (CLIA). Testing of 8 clinical samples for the Qualitative/Quantitative determination and compared side by side with the competitor. The assay was tested on Dia. Pro instrument named SARA.
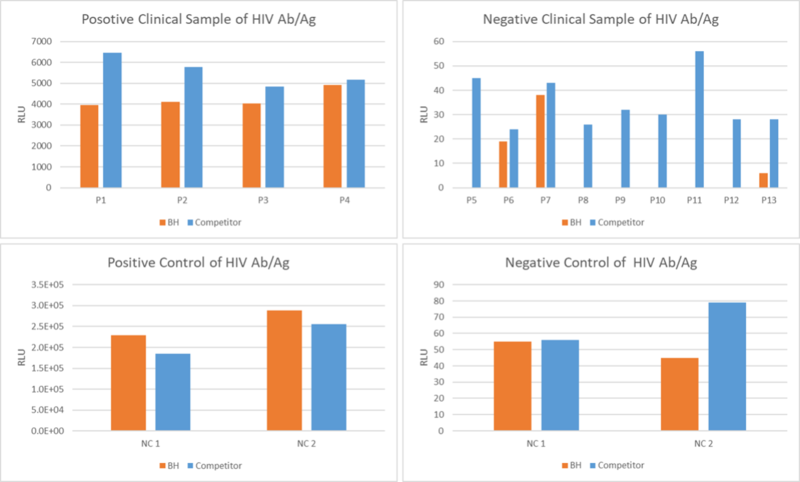
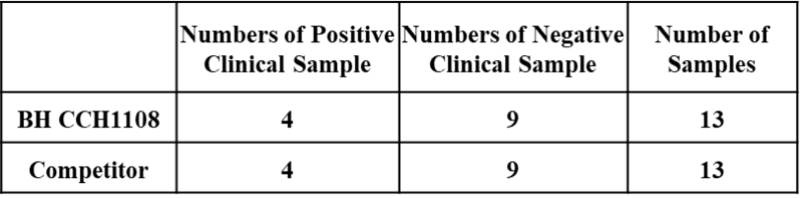
Figure 3. An assay was performed to assess the HIV Ab/Ag clinical sample by chemiluminescence immunoassay (CLIA). Testing of 13 clinical samples for the Qualitative/Quantitative determination and compared side by side with the competitor. The assay was tested on Dia. Pro instrument named SARA.
| Name | Download |
|---|